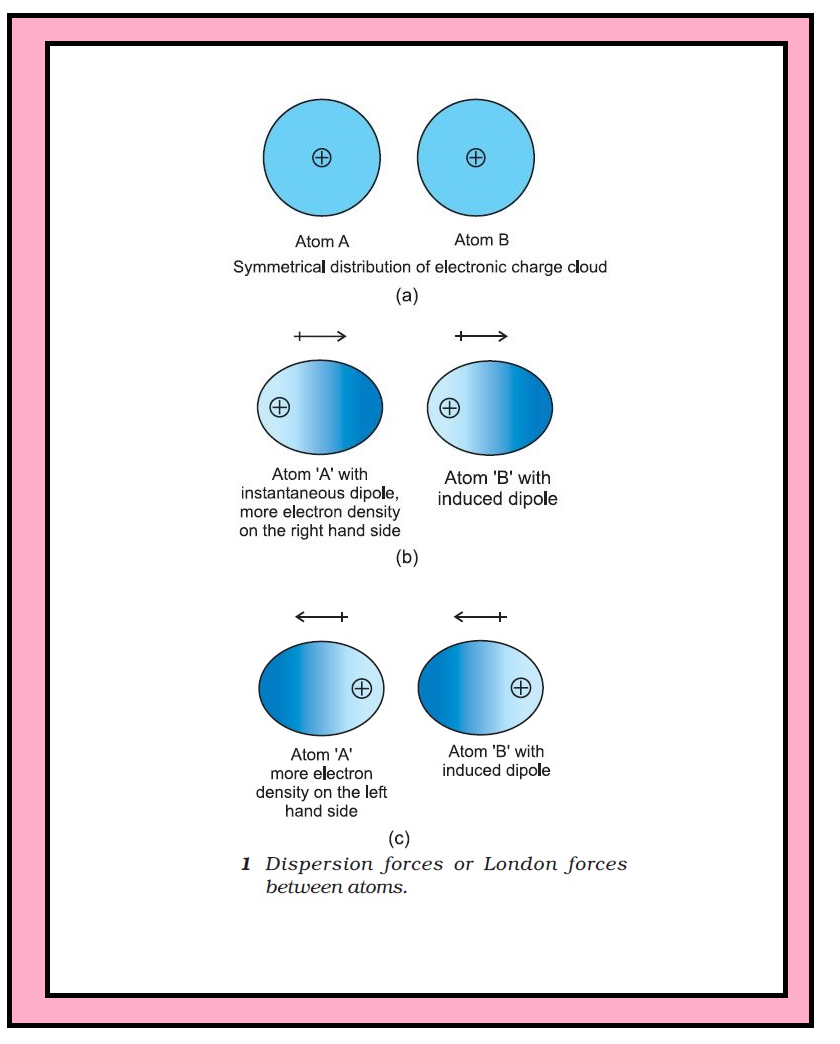
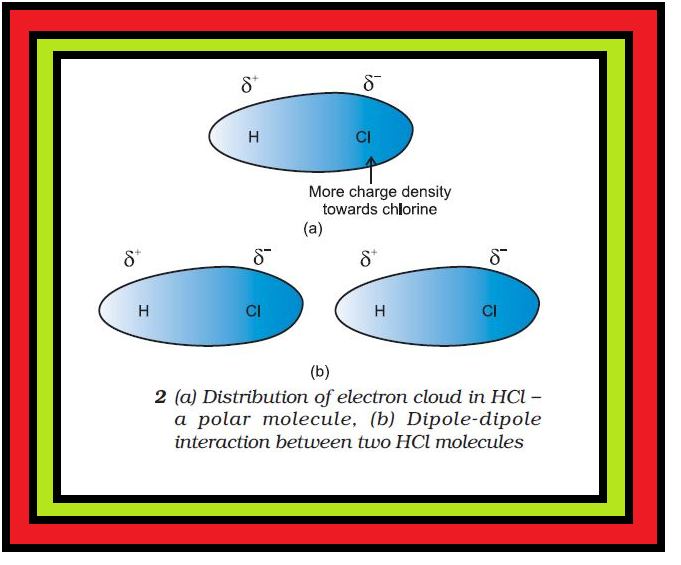
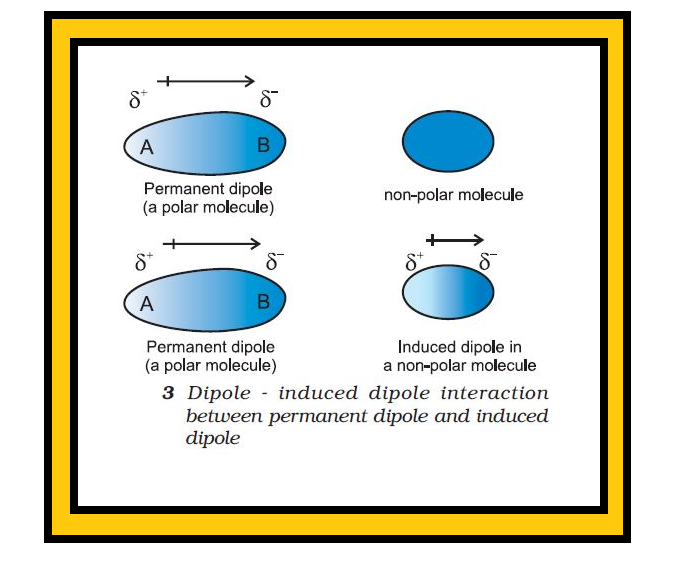
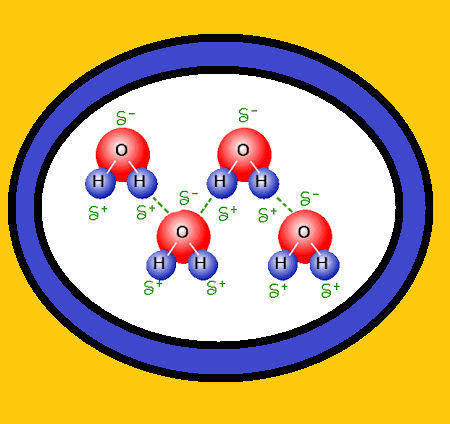
Introduction :
`=>` Most of the observable characteristics of chemical systems represent bulk properties of matter, i.e., the properties associated with a collection of a large number of atoms, ions or molecules.
● For example, an individual molecule of a liquid does not boil but the bulk boils.
`=>` Collection of water molecules have wetting properties; individual molecules do not wet.
`=>` Water can exist as ice, which is a solid; it can exist as liquid; or it can exist in the gaseous state as water vapour or steam.
● Physical properties of ice, water and steam are very different.
● In all the three states of water, chemical composition of water remains the same i.e., `H_2O`.
`=>` Characteristics of the three states of water depend on the energies of molecules and on the manner in which water molecules aggregate. Same is true for other substances also.
`=>` Chemical properties of a substance do not change with the change of its physical state; but rate of chemical reactions do depend upon the physical state.
`=>` Many times in calculations while dealing with data of experiments we require knowledge of the state of matter.
● Therefore, it becomes necessary for a chemist to know the physical laws which govern the behaviour of matter in different states.
`color{purple}♣ color{Violet} " Just for Curious"`
•The temperature at which all the three phases exist together is called triple point.
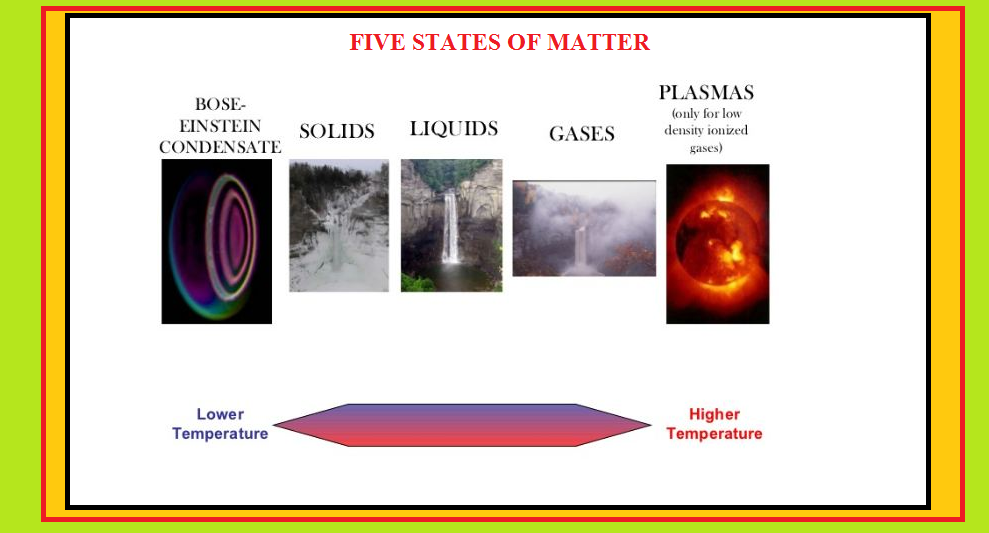
•Besides the three states of matter, there exists fourth state called plasma state(mixture of electrons and positively charged ions formed due to superheating of the gaseous state,e.g., in the sun or stars) and fifth state of supercooled solid in which atoms lose their identity and condense to form a single atom.
● For example, an individual molecule of a liquid does not boil but the bulk boils.
`=>` Collection of water molecules have wetting properties; individual molecules do not wet.
`=>` Water can exist as ice, which is a solid; it can exist as liquid; or it can exist in the gaseous state as water vapour or steam.
● Physical properties of ice, water and steam are very different.
● In all the three states of water, chemical composition of water remains the same i.e., `H_2O`.
`=>` Characteristics of the three states of water depend on the energies of molecules and on the manner in which water molecules aggregate. Same is true for other substances also.
`=>` Chemical properties of a substance do not change with the change of its physical state; but rate of chemical reactions do depend upon the physical state.
`=>` Many times in calculations while dealing with data of experiments we require knowledge of the state of matter.
● Therefore, it becomes necessary for a chemist to know the physical laws which govern the behaviour of matter in different states.
`color{purple}♣ color{Violet} " Just for Curious"`
•The temperature at which all the three phases exist together is called triple point.
•Besides the three states of matter, there exists fourth state called plasma state(mixture of electrons and positively charged ions formed due to superheating of the gaseous state,e.g., in the sun or stars) and fifth state of supercooled solid in which atoms lose their identity and condense to form a single atom.